In traditional compounding pharmacies, each medication can vary in potency, purity, and sterility. Not at Epicur Pharma®.
As an FDA-registered 503B outsourcing facility, we manufacture drugs with reliable quality, potency, and sterility from batch to batch. We’re manufacturing safer medications so you can prescribe with confidence—every time.
Epicur Pharma brings a unique advantage to the veterinary industry—consistency and reliability in every medication.
A division of Stokes Healthcare and a sister company of Stokes Pharmacy, Epicur® was developed in 2016 to align with the Food and Drug Administration’s (FDA) recommended guidelines and requirements for producing high-quality manufactured drugs.
The 503B standards put in place reflect current Good Manufacturing Practices (cGMP), the same regulations in place for human pharmaceuticals.
Epicur is dedicated to raising the standards in animal healthcare. We offer one of the largest selections of manufactured medications that are traditionally compounded. Our commitment to drug consistency and quality emphasizes our devotion to patient safety.
Watch the videos below to learn more about what makes us a different breed and how we keep our commitment to quality.
*does not apply to all products
Epicur Pharma® is currently licensed as an outsourcing facility in nearly all 50 states!
When purchasing from an FDA Registered 503B Outsourcing Facility, dispensing and unlimited hospital administration is permitted*.
*Varies based on individual state law. Federal law allows dispensing and administration – FDA Federal SEC. 503B. [21 U.S.C. 353b]
Ohio – The Ohio Board of Pharmacy has updated office stock and dispensing guidance for veterinarians, meaning the 7-day supply limitation does NOT apply to compounded drugs purchased from a 503B outsourcing facility. Read more about the updates! Epicur Pharma is licensed as a 503B Outsourcing Facility in Ohio. Start your order!
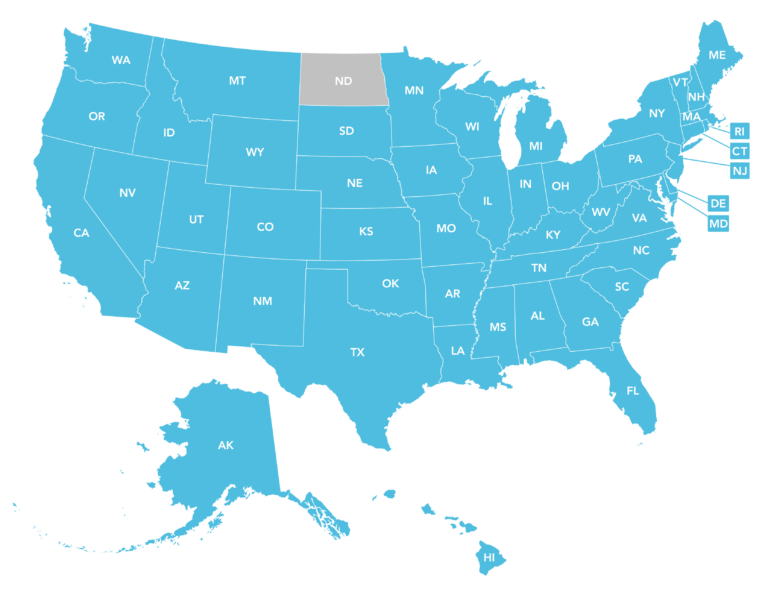
Current 04/2024
Stokes Healthcare has prepared medications for your individual patients since 1975 through Stokes Pharmacy. Recognizing the evolving needs of veterinary practices as regulations and
laws changed, including GFI 256 and USP guidelines, we expanded to include Epicur Pharma to serve the needs of many of your patients with standardized and tested bulk medications.
Each of our brands operates with key differences that impact your patient care and influence treatment plans, but they are all unified in their commitment to quality and improving patient outcomes with medication you trust. You can be confident in your patient care when you partner with us, knowing there is consistency and reliability in every medication.
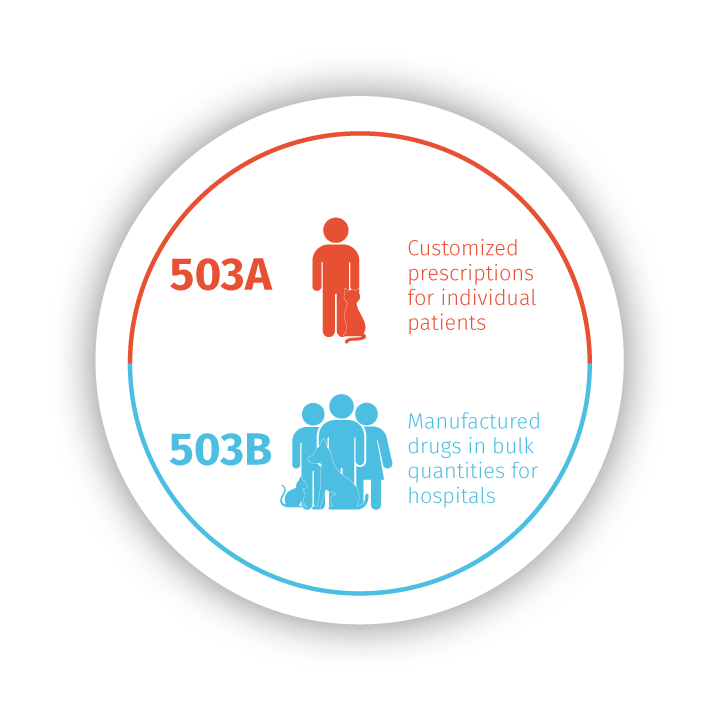
Get more details about Epicur’s 503B manufactured products and how they can make a difference in patient outcomes.
Contact Us
More to Explore
All information provided on this site is for educational purposes only. Every effort has been made to provide accurate and up-to-date information. It is not intended to be used as medical advice for the diagnosis of health problems nor does it include all possible uses, actions, safety measures, side effects, or interactions. Please consult a healthcare provider or veterinarian for the diagnosis and treatment of any health conditions. Epicur Pharma® manufactured veterinary orders are for non food chain animals only. Epicur Pharma® is registered with the FDA as an outsourcing facility.
© 2024 Epicur Pharma®
Our website uses cookies to enhance your browsing experience. By clicking “Accept Cookies” you are agreeing to our use of cookies. If you select “No Cookies” we will respect your privacy, though your browsing experience may be limited.